Rank the Following Alcohols From Strongest to Weakest Acid
See is too or its end. Let STRONGEST 1 and WEAKEST 7.
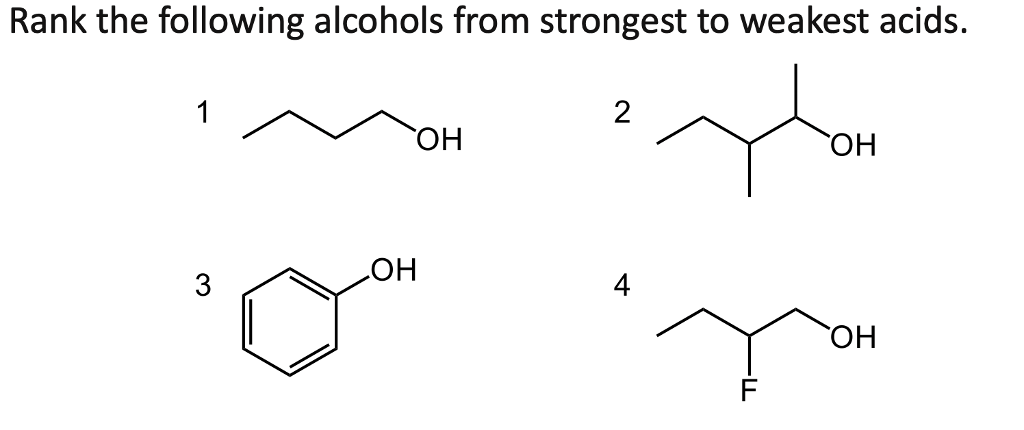
Solved Rank The Following Alcohols From Strongest To Weakest Chegg Com
A single bond between two carbon atoms with different hybridizations has a small dipole.
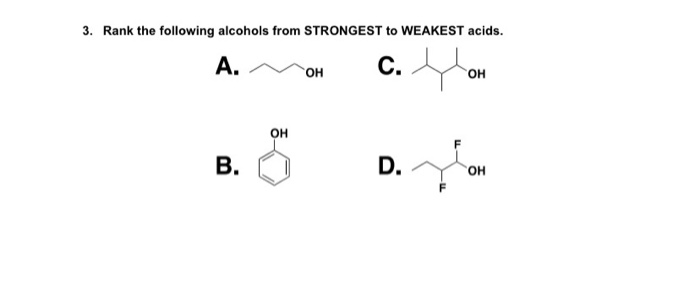
. Rank the following alcohols from strongest to weakest acid. IIIVIIII IIThe nitro group is a strong electron-withdrawing group and will stabilize the. How does the presence of an electronegative substituent such as mathrmCl affect the acidity of a carboxylic acid.
Up to 256 cash back Get the detailed answer. Rank the following acids in order of increasing weakest to strongest acidity. Find step-by-step Chemistry solutions and your answer to the following textbook question.
Rank the following alcohols from strongest to weakest acid. Which molecule contains both an alcohol and a ketone functional group. Here we can can sue do the second carbon from or is group.
Its too or edge greater then see is too. How does the presence of an electronegative substituent such as CI affect the acidity of a carboxylic acid. Rank the acids shown in decreasing strongest to weakest order of acidity OH CL BR Conversion to the acid chloride followed by treatment with LIALHOCCH333 Acids can be.
Explain the relative acidities. This is the best answer based on feedback and ratings. For each compound indicate the atom that is most apt to be protonated.
The strongest acid among the following aromatic compounds is. Unser for Barbie Prisons off and Elektronik get you subsidence kin a foot The acidity of the compound. Click hereto get an answer to your question Rank the following compounds in order of increasing acidity weakest acid first.
So order off ascetics Ekeren. C H 2 C H C H 2 O H C H 3 C H 2 C H 2 O H H C C C H 2 O H CH_2CHCH_2OH CH_3CH_2CH_2OH HCCCH_2OH C H 2 C H C H 2 O H C H 3 C H 2 C H 2 O H H C CC H 2 O H. Rank the following alcohols from strongest to weakest acid and explain relative acidities.
Carboxylic acid Phenol Water Alcohol. Answer for the question. For the following list of acids rank the acids in strength from weakest acid to strongest acid.
Rank the following acids in order of bartleby. Thus the final acidic order of the discussed compounds is. Rank the following alcohols from strongest to weakest acid.
See its two or edge greater than see is three. CH_2CHCH_2OH CH_3CH_2CH_2OH HCCCH_2OH. On comparing the acidity of carboxylic acid phenol and alcohol Carboxylic acids are stronger acids than corresponding alcohols and even phenols because it loses its proton to form a stable conjugate base.
Rank the following acids in decreasing strongest to weakest order of acidity. Explain the relative acidities. Rank the following alcohols from STRONGEST to WEAKEST acids.
Click hereto get an answer to your question Rank the following compounds in order of increasing acidity weakest acid first. F3COH 1 See answer Advertisement Advertisement dariusardelean558 is waiting for your help. Answer for birdie or did off necessary.
For the following list of acids rank the acids in strength from weakest acid to strongest acid. Rank the following carboxylic acids from strongest to weakest acid. Rank the following compounds in order of increasing.
For the following list of acids rank the acids in strength from weakest acid to strongest acid. Solve Study Textbooks Guides. Join Login Class 12 Chemistry Alcohols Phenols and Ethers Chemical Reactions of Alcohols and Phenols Rank the following compound.
Citrin is its C C C. Rank the following alcohols from strongest to weakest acid. CCl_3CH_2OH Ka57510 -13 CC l3.
Explain the relative acidities. 1 3 2 Rank the following carboxylic acids from strongest to weakest acid. C C l 3 C H 2 O H K a 575 1 0 13.
Rank the following carboxylic acids from strongest to weakest acid. Join Login Class 12. COH O OCH 3 COH O NO 2 COH O CH 3 COH O Cl I II III IV Ans.
K_a 575 times 10-13 K_a 129 times 10-13 K_a 490 times 10-13 b. For the following list of acids rank the acids in strength from weakest acid to strongest acid. Rank the following carboxylic acids from strongest to weakest acid.
Add your answer and earn points. Select HF Select CH3CH2NH2 Select HI Select CH3CCH Select CH32CCH OH Select CH3CO2H Select CH3CH2CH2CH3. Hi year the get a will you said stronger.
What is the direction of the dipole in the indicated bonds. We have Hear about it off isnt exit twenties c. 2 1 3 Rank the following compounds from strongest to weakest acid.
How does the location of the substituent affect the acidity of the carboxylic acid.
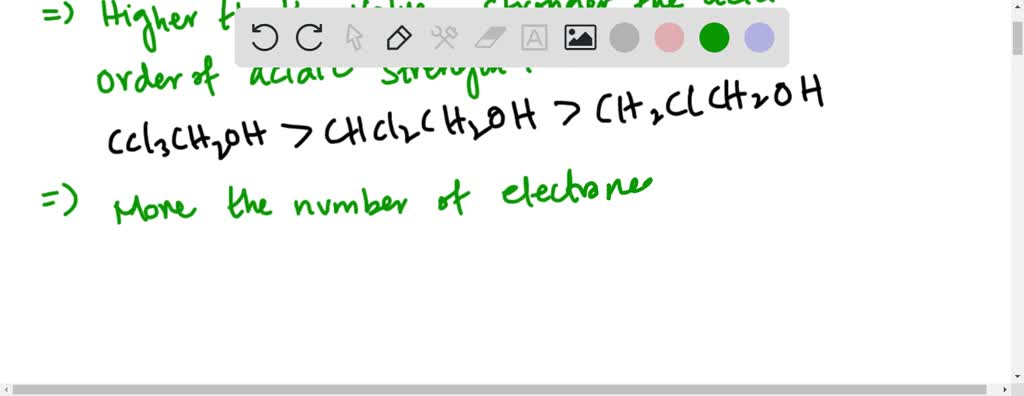
Solved A Rank The Following Alcohols From Strongest To Weakest Acid Mathrm Cl 3 Mathrm Ch 2 Mathrm Oh K Mathrm A 5 75 Times 10 13 Mathrm Ch 2 Mathrm Clch 2 Mathrm Oh K Mathrm A 1 29 Times 10 13 Mathrm Chcl 2

Pin On Things That Make Life Easier

Solved 3 Rank The Following Alcohols From Strongest To Chegg Com
Comments
Post a Comment